A combined desalination–electrolysis system that can produce green hydrogen directly from seawater has been developed by a team in China. This integrated process uses a low-energy method to purify seawater, making it one of the first viable approaches to use salt water as a source of hydrogen. The purification step uses phase transitions to remove impurities and could have additional applications in wastewater treatment and resource recovery.
Splitting water with electricity has been experimented with for over 200 years and the reactions involved are well-understood: at the cathode, H+ ions gain electrons to form hydrogen gas whilst OH- loses electrons at the anode to form oxygen. But despite the simplicity of the underlying chemistry, effective electrolysis is a particularly complicated process. Water splitting is thermodynamically unfavourable and requires both specifically designed catalytic electrodes and a significant input of energy to drive the reaction. Even trace impurities can damage the delicate structure of the cell, leading to membrane pores becoming blocked, expensive electrodes corroded and unwanted byproducts formed.
Chloride ions in seawater are a particular problem and undergo competing oxidation at the anode to produce chlorine. Not only does this side reaction reduce the electrochemical efficiency of the cell, but chlorine is an extremely corrosive gas which rapidly degrades the electrodes and inactivates the cell. ‘Approaches to suppress corrosion by coating catalysts have had modest success,’ explains Heping Xie, an energy chemist at Shenzhen University in China. ‘But the composition of seawater changes [with] location, season [and] human behaviour so electrolysers can’t be universally compatible.’ With an average salt concentration of around 3.5%, the chloride content of seawater makes direct electrolysis unfeasible.
‘Desalinating seawater before electrolysis can eliminate problems,’ says Zongping Shao, an electrocatalytic chemist at Nanjing Tech University in China. ‘But [it] requires additional energy and space [so it’s] less economically and practically attractive.’ Currently, the energy cost of desalination outweighs the value of hydrogen generated by electrolysis. However, the abundance of seawater, coupled with the urgent need for green fuels is motivating researchers to find innovative solutions to these problems.
Splitting seawater
By harnessing the purifying power of evaporation, Xie and Shao have developed the first practical and scalable seawater electrolysis system. Their in situ purification system uses a liquid–gas–liquid phase transition to generate pure water from seawater directly within the electrochemical cell, a process driven by the subsequent electrolysis.
A porous PTFE-based membrane separates seawater from the inside of the cell, with the high density of fluorine atoms creating a hydrophobic barrier impervious to water and its impurities but permeable to water vapour. On the other side, a concentrated potassium hydroxide solution surrounds the electrodes and provides the driving force for the water vapour migration. ‘The potassium hydroxide electrolyte is at a higher concentration than the salt concentration in the seawater ,’ explains Alexander Cowan, a sustainable fuels researcher at the University of Liverpool, UK. ‘The resultant difference in water vapour pressure (as a result of the salt gradient) leads the water from the seawater side to pass through the membrane and into the potassium hydroxide solution.
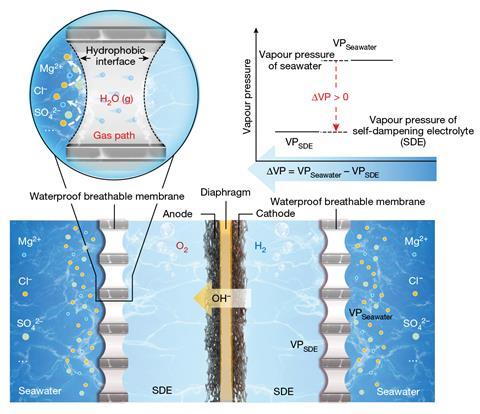
Left in isolation, this system would ultimately reach an equilibrium and water migration would stop as the concentrations on each side of the membrane became equal. However, the consumption of purified water by the electrolysis reaction provides a continuous driving force and maintains the concentration gradient across the membrane. By altering the rate of water migration or electrolysis, the system effectively self-regulates and ensures the pure water is used as fast as it’s produced. ‘It can actually be considered as a dynamic balance system,’ explains Xie. ‘If the initial electrolysis rate is higher than the water migration rate, the electrolyte concentration increases, leading to an increase in the water vapour pressure difference and consequently, the water migration rate increases.’
Following successful laboratory trials, the team were keen to demonstrate the practicality of this approach at scale and installed a demo device in Shenzhen Bay. The compact unit ran for an initial test period of 133 days producing over a million litres of hydrogen without any evident corrosion of the catalyst or increase in impurities. ‘It’s a nice demonstration of the technical feasibility of carrying out direct seawater electrolysis for prolonged periods without any apparent loss in activity,’ says Cowan. ‘A challenge will be to develop a device which can achieve a significant decrease in operating potentials in order to make them comparable to more conventional membrane electrolysers.’
‘It solves a longstanding technical bottleneck in this field,’ observes Xuping Sun, an electrocatalysis researcher at the Shandong Normal University in China. ‘But [it still] needs further development. To make seawater electrolysis systems more relevant to industrial applications, higher current densities are necessary.’ Xie and Shao are eager to develop the device for industrial use and are currently investigating ways to reduce the energy consumption and improve the performance of the catalysts. ‘This technology has great potential,’ says Shao. ‘[We hope that] people can use this liquid–gas–liquid phase transition mechanism in other fuel production and resource recovery fields.’
References
H Xie et al, Nature, 2022, DOI: 10.1038/s41586-022-05379-5
















No comments yet